Two new vaccines for respiratory syncytial virus (RSV) are available this year for people aged 60 and older, as well as for those between 32 and 36 weeks pregnant.
These vaccines-Arexvy by GSK and Abrysvo by Pfizer—are the first for RSV.The new vaccines come with common vaccine side effects including swelling or pain at the injection site, fever, and headache, among others.
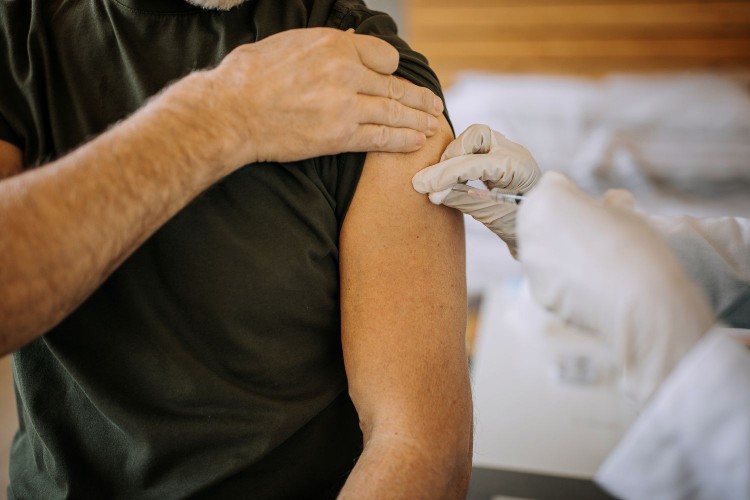
Two vaccines, Arexvy (GSK) and Abrysvo (Pfizer), have already rolled out; both are single dose and approved for people aged 60 and older, and Abrysvo has also been approved for those between 32 and 36 weeks pregnant to help protect newborns. These are the first RSV vaccines ever available.
These are “great [tools] to protect some of our most vulnerable patients,” Nora Colburn, MD, an infectious diseases specialist and clinical assistant professor at The Ohio State University Wexner Medical Center, told Health.
If you are eligible for these vaccines, you might be wondering if it makes sense to get a shot—and, more specifically, if it will come with side effects. Here’s what you need to know.
While all vaccines have the same end goal, manufacturers design them in different ways, Brian Labus, PhD, MPH, REHS, an expert in infectious diseases and an assistant professor in the department of epidemiology and biostatistics at the UNLV School of Public Health, told Health.
Because subunit vaccines use only certain proteins from the germ, they give a “very strong” immune response that’s targeted to key parts of the virus, according to the Department of Health and Human Services. Other subunit vaccines include those protecting against HPV, hepatitis B, and shingles.
Are the Vaccines Effective?
“Both vaccines were 80% to 90% effective in reducing RSV illness during the first season after vaccination,” Labus said.
However, he noted that “the clinical trials were not large enough to determine the efficacy in reducing hospitalization or death.”
As for which vaccine was more effective, William Schaffner, MD, a spokesperson for the National Foundation for Infectious Diseases (NFID), told Health that there is no substantial difference between Arexvy and Abrysvo. He recommended getting the one your medical provider has stocked.
According to Labus, adults over age 60 may have an elevated risk of RSV complication if they live in a long-term care facility or have chronic conditions that make it harder for them to fight off an infection, such as heart, lung, liver, or kidney disease.
“Unlike other vaccines, there isn’t a blanket recommendation that everyone eligible receive the vaccine,” he said. “The CDC has emphasized the importance of shared clinical decision-making in using the RSV vaccine, where patients and their healthcare providers determine if the person will benefit from the vaccine.”
Dr. Colburn said it’s possible to get the RSV vaccine at the same time as the COVID and flu vaccines, but it’s best to discuss whether to do so with a healthcare provider. “We just don’t have much data on how the three interact in terms of efficacy or side effects,” Labus said.




:max_bytes(150000):strip_icc()/GettyImages-1807240274-2c7c39cb23f34a97bb2d8620911c343d.jpg)



